At the University of Sussex, we are building a clock based on a trapped molecular nitrogen ion to search for physics beyond the standard models of particle physics and cosmology.
Nitrogen ions are very good candidates for measuring potential changes in the fundamental constants because they have vibrational and rotational transitions that are sensitive to changes in the proton-to-electron mass ratio, but insensitive to changes in their environment. Precision spectroscopy of these vibrational transitions has the potential to achieve a fractional frequency uncertainty below 10-20, which is better than the best atomic clocks to date.
In our experiment, we will probe a vibrational transition in the electronic ground state of the molecular ion. To perform spectroscopy on this transition, a single nitrogen ion will be trapped in an ion trap alongside a calcium ion, which is needed for cooling and detection.
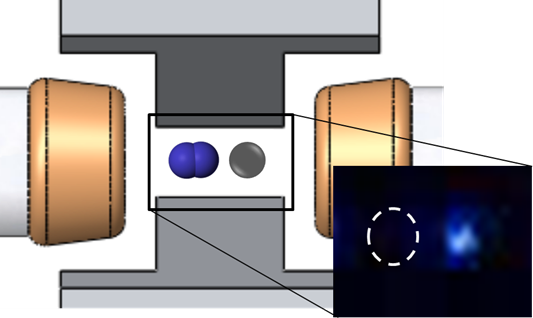
Figure 1 Drawing of ion trap with a nitrogen molecular ion and calcium atomic ion. Inset, a real image of a calcium and nitrogen ion in the trap.
The ion trap allows a high level of localisation and control of the ions and is under ultra-high vacuum to prevent collisions with background gas. The figure below shows the set-up of the clock system, consisting of a vacuum chamber for the ion trap and an attached molecular beam line.
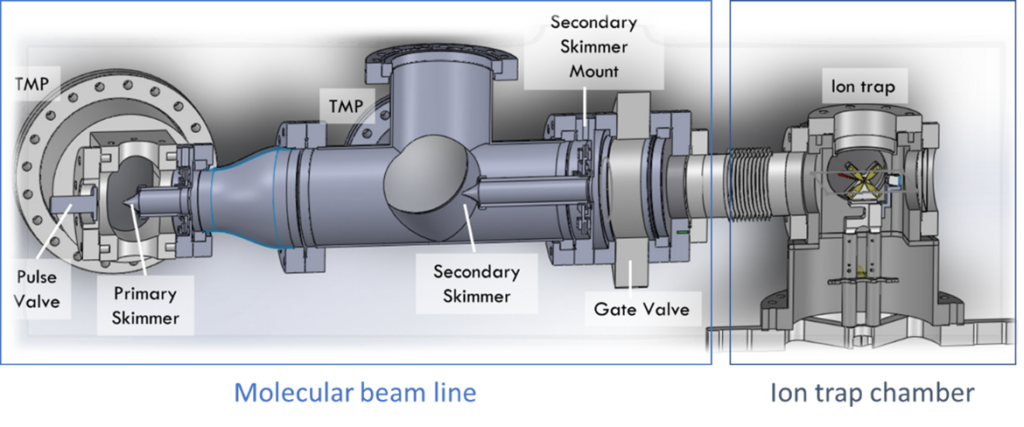
Figure 2 Set-up of the ion trap and molecular beam line.
To load nitrogen ions into the trap, a supersonic beam of neutral nitrogen gas is injected into the ion trap where it is ionised with an intense laser pulse. Through the Coulomb repulsion between the nitrogen and calcium ions, the nitrogen is cooled to ~500 uK through laser cooling of the calcium ion. In addition to cooling the nitrogen ion, the calcium ion facilitates the ‘read-out’ of its internal state via quantum logic spectroscopy. This is necessary due to the lack of cycling transitions within the nitrogen ion.
More information can be found here.
